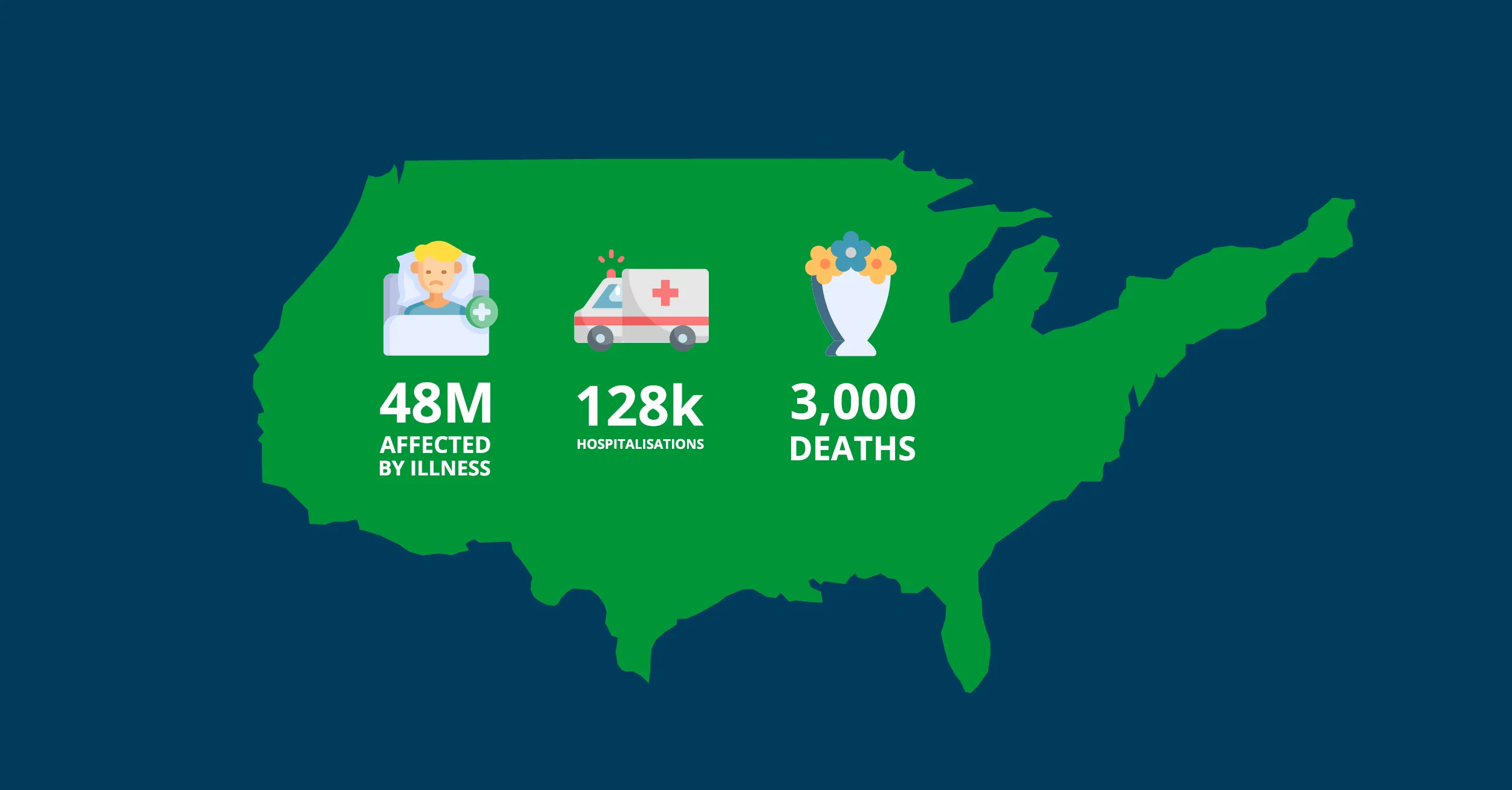
Each year in the US, approximately 48 million people are affected by foodborne illnesses, resulting in some 128,000 hospitalisations and 3,000 deaths. These figures form a significant public health burden but are largely preventable with appropriate traceability measures.
With the latest updates to the Food Safety Modernization Act (FSMA), the FDA aims to dramatically improve food safety in the US by shifting the focus to preventing foodborne illnesses by enhancing data-driven transparency and accountability within the food system.
What is FDA Traceability Rule FSMA 204?
Section 204 of the FDA Food Safety Modernization Act (FSMA 204), “Requirements for Additional Traceability Records for Certain Foods”, adds traceability record-keeping requirements for businesses that manufacture, process, pack, or hold certain high-risk food products.
Which food businesses must comply with FSMA 204?
FSMA 204 affects any business that manufactures, processes, packs, or holds food items covered under the Food Traceability List. Under the legislation, non-US companies handling products for final consumption in the US will also have to follow traceability requirements as companies located in the US.
The following list provides an overview of the types of fresh produce and ingredients included on the Food Traceability List:
- Cheeses
- Fresh, unprocessed eggs
- Nut butters
- Fresh fruits and vegetables
- Fish and shellfish
- Ready-to-eat deli salads
A full version of the Food Traceability List is available directly from the FDA
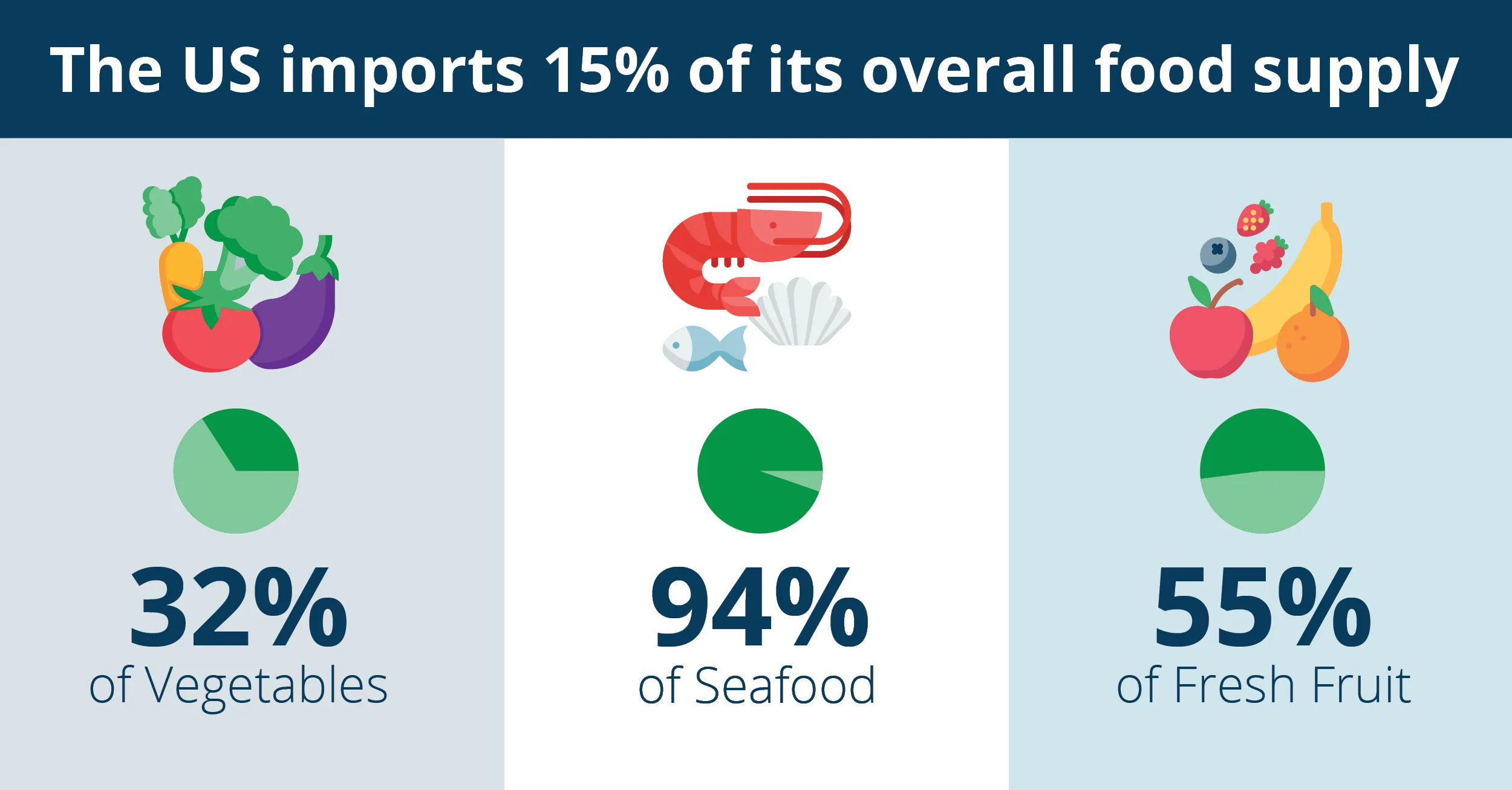
What are the key requirements of FSMA 204?
FSMA 204 specifies the need for businesses to maintain and process data relating to Critical Tracking Events (CTEs) that reflect stages within the supply chain at which food safety incidents are most likely to occur. Tracking these events makes it possible to identify the source of contamination, food safety, or quality issues that may arise during food’s journey from farm to fork and allows for rapid response when an incident is identified.
The CTEs at which point data needs to be acquired include:
- The harvesting and cooling of fresh produce before distribution
- Initial packing of produce to make it suitable for transportation
- Shipping and receiving of produce at each stage of the supply chain
- The stage at which raw ingredients are transformed into consumer-ready products
Businesses will be required to keep records containing Key Data Elements (KDEs). The exact information required varies depending on the CTE. However, traceability lot codes will be central to the proposed requirements, helping to identify foods as they move through the supply chain.
A comprehensive list of the specific data elements required for each tracking event is available directly from the FDA.
Affected organisations will need to put systems in place to collect data at each CTE; all data will need to be shared with supply chain partners and retained for two years following the sale or distribution of a food item. All traceability information must also made accessible to the FDA, in electronic format, where required, within 24 hours of being requested. Non-compliance can lead civil action all the way up to criminal prosecution.
What is the timeline for compliance with FSMA 204?
FSMA 204 officially took effect in January 2023. Affected organisations were given a three-year window to meet the requirements, with a final deadline set for 20th January 2026. This extended timeline allows for thorough preparation and implementation of traceability solutions across the supply chain.
How can companies prepare for the FSMA 204 compliance date?
Companies can ensure that their traceability record-keeping adheres to FSMA 204 guidelines by collecting all necessary KDEs and capturing this data as part of wider coding and marking efforts. Exact requirements will vary based on specific product types, and businesses should consult the complete list of KDEs as provided by the FDA.
The first step will be to identify gaps in capturing data across the supply chain and work with supply chain partners to record all necessary data. Data recording will require multiple systems to communicate, so companies will need to consider coding and marking systems that allow for interconnectivity between other equipment. Companies should look for partners who provide flexible capabilities, using industry standards for data transfer, to allow all necessary software components and systems to track and record data across the supply chain.
The next step will be to ensure that the final consumer product can be tied back to the data required by the FDA using variable data printing. Companies already adding variable data onto products and packaging, such as date codes, may only require a small change to their current coding and marking setups to include batch and lot information. One suggested way this variable data element can be implemented is through a scannable 2D barcode (or 2D code), such as a QR code.
While a scannable 2D code is not a requirement of the FSMA 204, this does present certain opportunities for businesses. Companies who look to incorporate FSMA traceability data within a QR code powered by GS1 can align FSMA compliance with the GS1 initiative to see 2D codes accepted at the point-of-sale.
By integrating multiple different initiatives at once, companies can avoid the risk of having to make additional changes further down the line. Furthermore, 2D codes are more resilient to damage or degradation through the supply chain. While a digit or character can be illegible with a slight smudge, a 2D barcode can be successfully decoded even with moderate damage to the barcode itself.
Aligning FSMA 204 requirements with the global move to 2D codes at the point of sale will not only benefit brands by helping to streamline changes and avoid additional interruption but will also make it easier for businesses to track through the supply chain and help to protect consumers from the risk of foodborne illnesses – a key aim of FSMA 204.
By including unique product data within a scannable 2D code, businesses can provide additional information to consumers, including automatic alerts when a code is scanned to advise of any potential food safety incidents or recalls which could make the food unsafe to eat.
What do companies need to consider when implementing variable data printing?
When implementing variable data printing, companies will need to ensure that the coding technology selected can match the speed and throughput of a production line and that the print quality is high enough to produce scannable 2D codes – but this is only part of the puzzle.
A key thing to consider when implementing variable data printing for any compliance exercise is that data needs to be accurate, or all the effort and cost of implementing new systems will be wasted. Human errors remain a significant issue in the global food value chain. In fact, in January 2024 alone, the FDA issued 19 recalls for food products in the US, 18 of which were due to undeclared allergens due to labelling mistakes.
Data accuracy is paramount when implementing variable data printing; the easiest way to avoid the risk of human error in data entry is to take manual labour out of the equation. Companies should look to implement a closed-loop code and check system to ensure that the information embedded in a scannable 2D code is correct at the time printing and that the code can be effectively scanned throughout the supply chain to the end consumer.
Businesses should partner with a trusted variable data solutions provider capable of offering a complete, closed-loop code and check system and making complying with FSMA 204 and any future traceability requirement seamless.
Find out more about Domino’s closed-loop variable data printing solutions here